- Effectivity
- Clinical Research
- Mechanisms of Action
- Health Claims
- Dosage
- Why choose AP BIO®?


Discover AP-Bio®
AP-Bio® is a fast-acting Andrographis paniculata with 7 bioactives, clinically researched to manage the symptoms of common cold at a low dose.

AP-Bio® works for you by effectively
lowering the symptoms of cold and flu
Cough
Earache
Headache
Sore throat
Fatigue
Fever
Sleep disturbance
Nasal discharge
Expectoration
AP-Bio® is backed by 3 clinical studies:
- Summary
- Clinical Study 1
- Clinical Study 2
- Clinical Study 3
Clinical Study 1
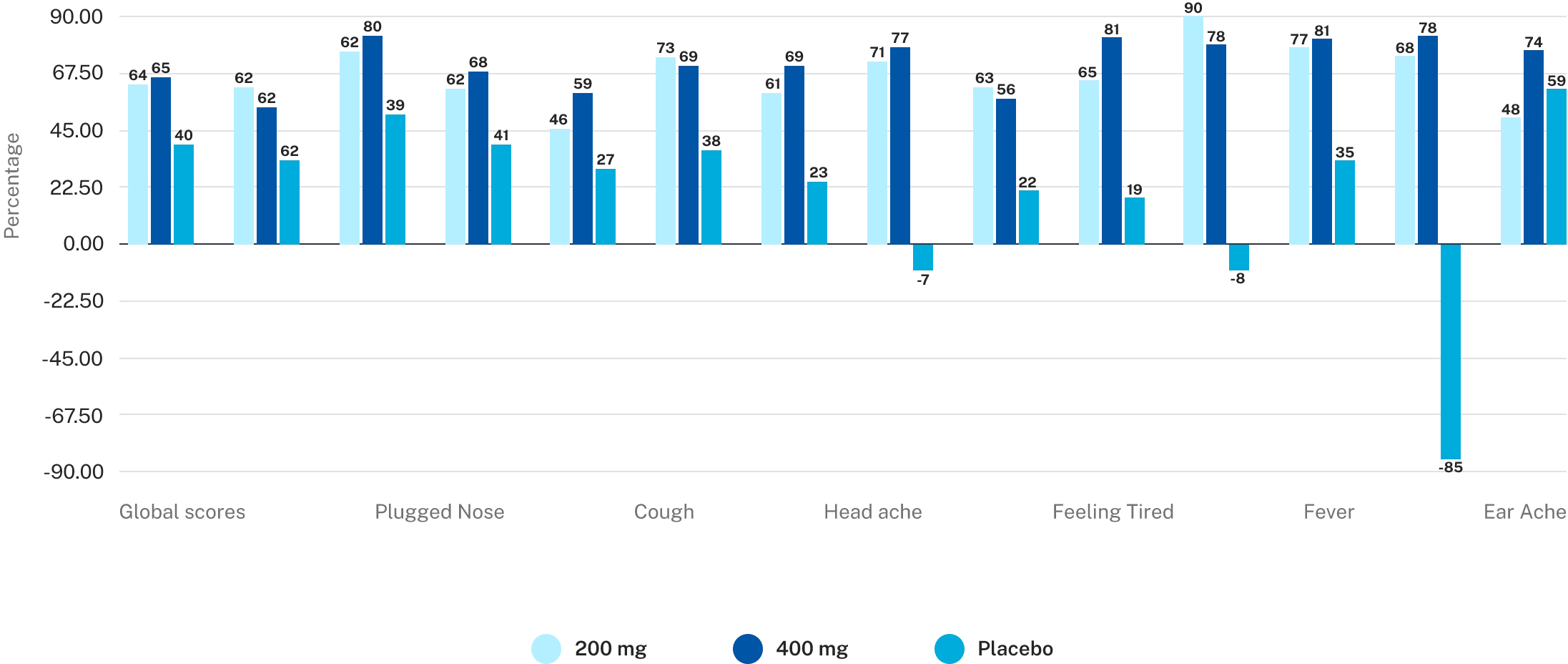
AP-Bio® 200 mg and 400 mg groups showed significantly better resolution of symptoms of uncomplicated upper respiratory tract infections when compared with placebo
Clinical Study 2
AP-Bio® showed 2 times efficacy as compared to placebo and was well tolerated
Clinical Study 3
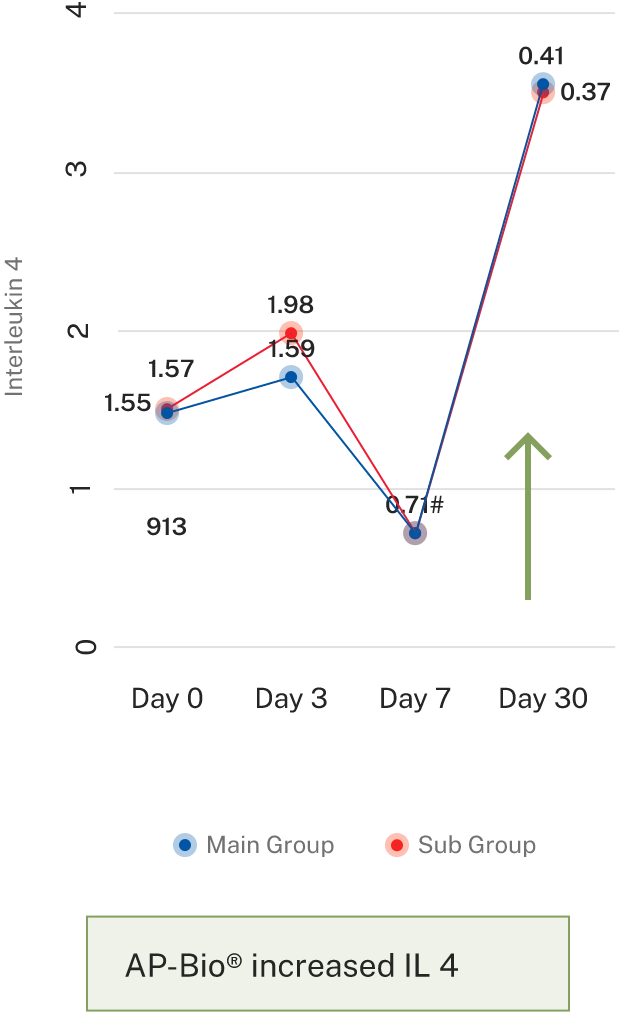
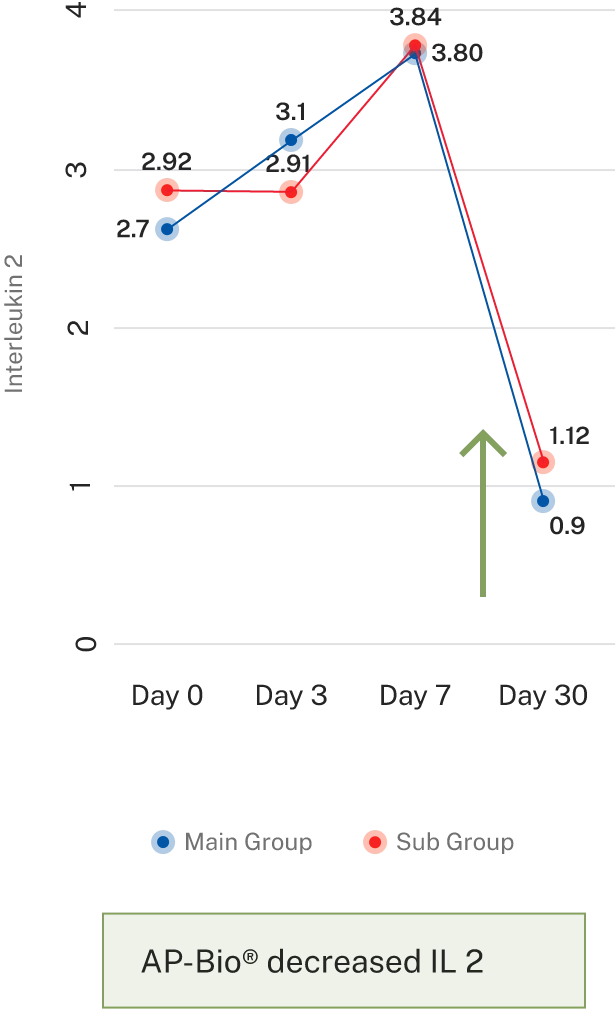
AP-Bio® supports immunity by increasing INF gamma, IL-4 coupled by increased CD4+ and absolute lymphocytes count and decreasing IL-2
Randomized double-blind placebo controlled clinical study of AP-Bio® - Healthy-inflammatory response
Condition
Subjects with symptoms of upper read more respiratory tract infection
Participants
300 adults (Age: 18-60 years)
Dose
200 mg and 400 mg
Duration
7 days
Evaluation
Baseline, 6 hours, Day 2, Day 3, Day 5 and Day 7
Outcome
AP-Bio® 200 mg and 400 mg groups showed significantly better read more resolution of symptoms of uncomplicated upper respiratory tract infections when compared with placebo
Jeffrey Pradeep Raj et. al., Complementary Therapies in Medicine 73 (2023) 102934, Efficacy and safety of AP-Bio® in participants with uncomplicated upper respiratory tract viral infection (common cold) − A phase III, double-blind, parallel group, randomized placebo-controlled trial, 2023
Know MoreRandomized double-blind placebo-controlled clinical studyof AP-Bio® - Healthy-inflammatory response
Condition
Subjects with symptoms of common cold
Participants
223 adults (Age: 18-60 years)
Dose
200 mg per day
Evaluation
Day 1, Day 3, and Day 5 (using Visual Analogue Scale)
Duration
5 days
Outcome
AP-Bio® showed 2 times read more efficacy as compared to placebo and was well tolerated
Saxena et. al., Phytomedicine 17 (2010) 178–185, A randomized double blind placebo-controlled clinical evaluation of extract of Andrographis paniculata in patients with uncomplicated upper respiratory tract infection
Know MoreRandomized double-blind placebo controlled clinical studyof AP-Bio® - Healthy-inflammatory response
Condition
Healthy volunteers
Participants
30 adults
Dose
100 mg twice a day
Evaluation
Screening day, day 3, day 7, day 30
Duration
30 days
Outcome
AP-Bio® supports immunity by read more increasing INF gamma, IL-4 coupled by increased CD4+ and absolute lymphocytes count and decreasing IL-2
Rajanna et. al., Journal of Ayurveda and Integrative Medicine 12 (2021) 529e534, Immunomodulatory effects of Andrographis paniculata extract in healthy adults e An open-label study
Know More
- Results – Subjective parameters
- Results-Objective parameters
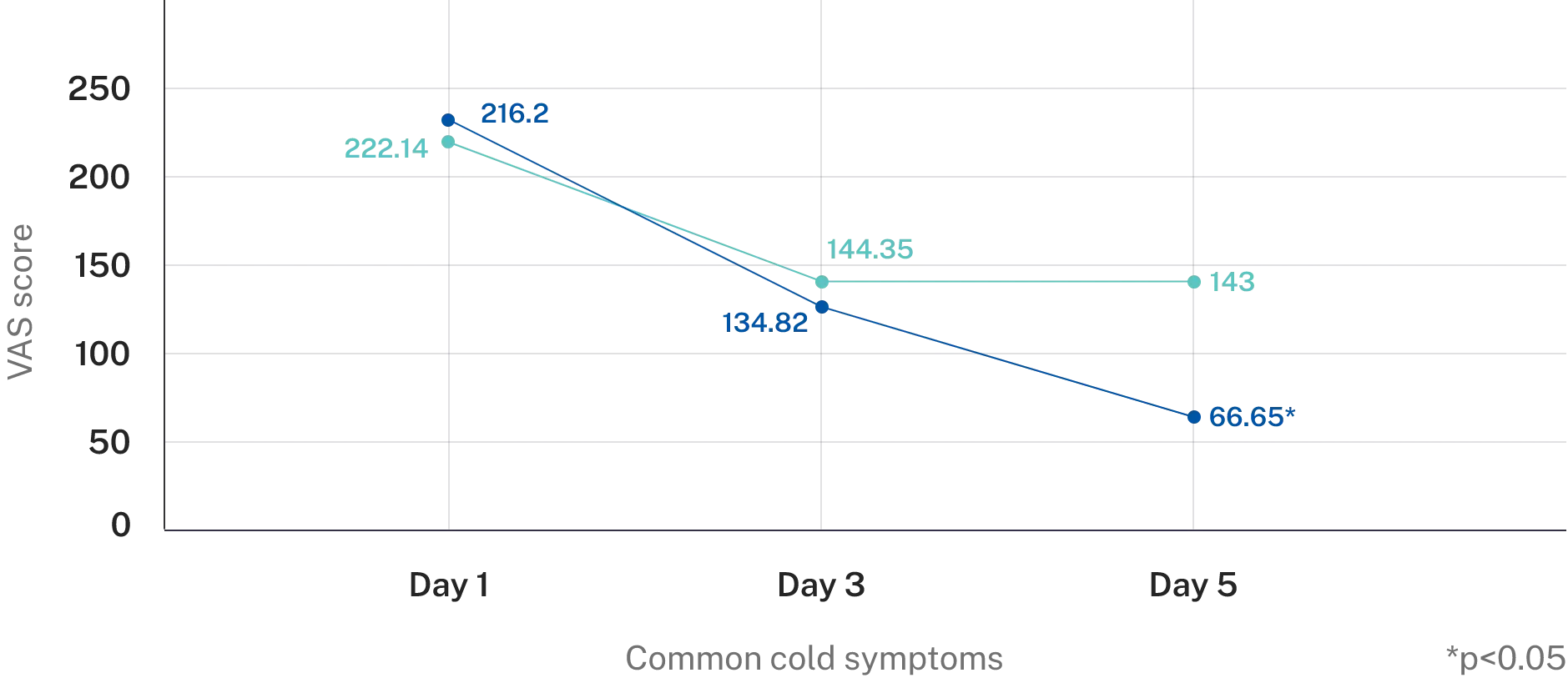
Reduction in common cold symptoms
- AP-Bio® showed prominent effects at day 3 and the efficacy is 1.6 times the placebo effects.
- Minimal clinical important difference was observed at day 3.
Effect of AP-Bio® on Nasal mucosal weight
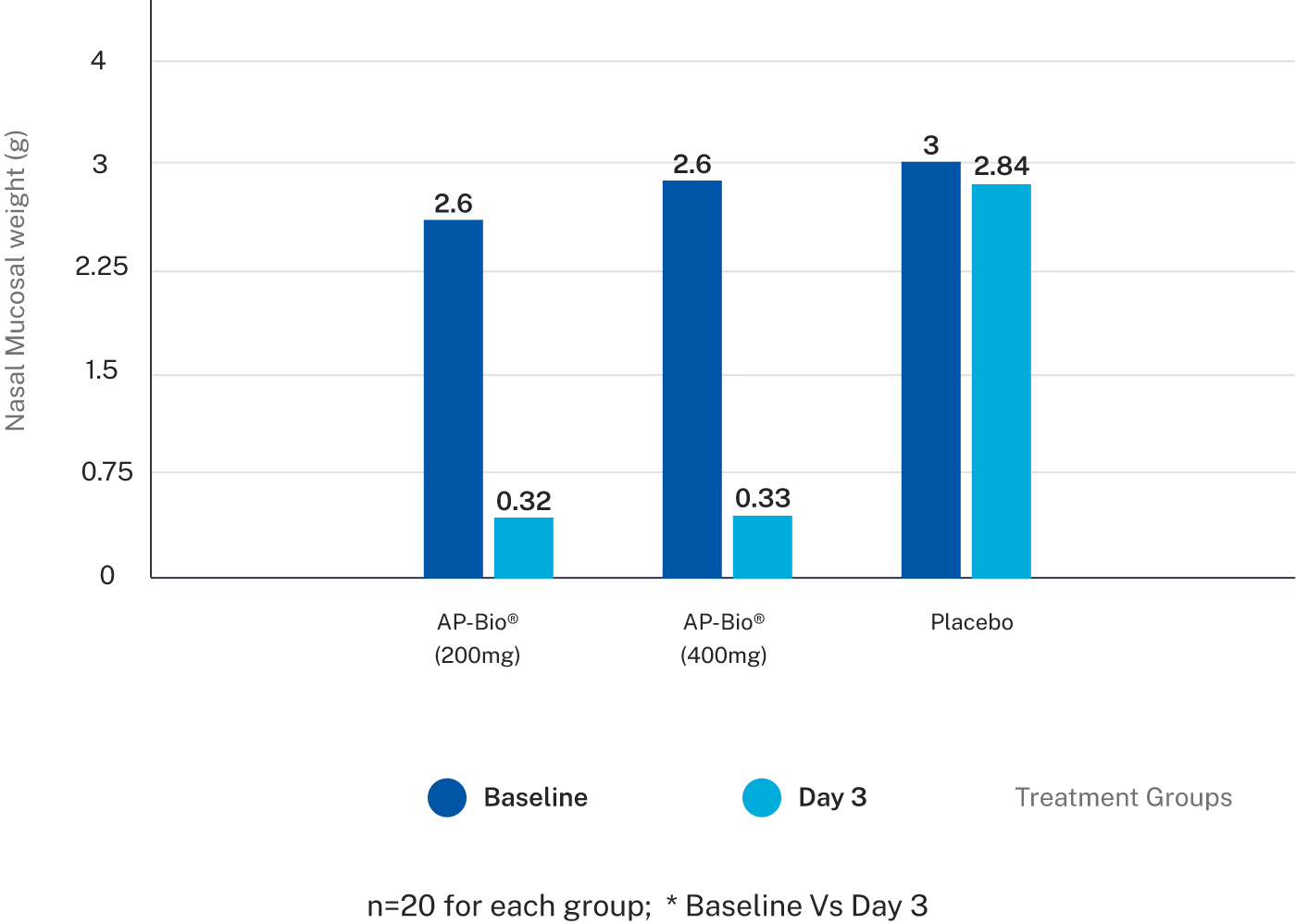
The objective parameters like nasal mucosal weight, number of tissue papers used showed a strong trend towards significant difference in both the AP-Bio® groups when compared to the placebo group.
Effect of AP-Bio® on Tissue counts
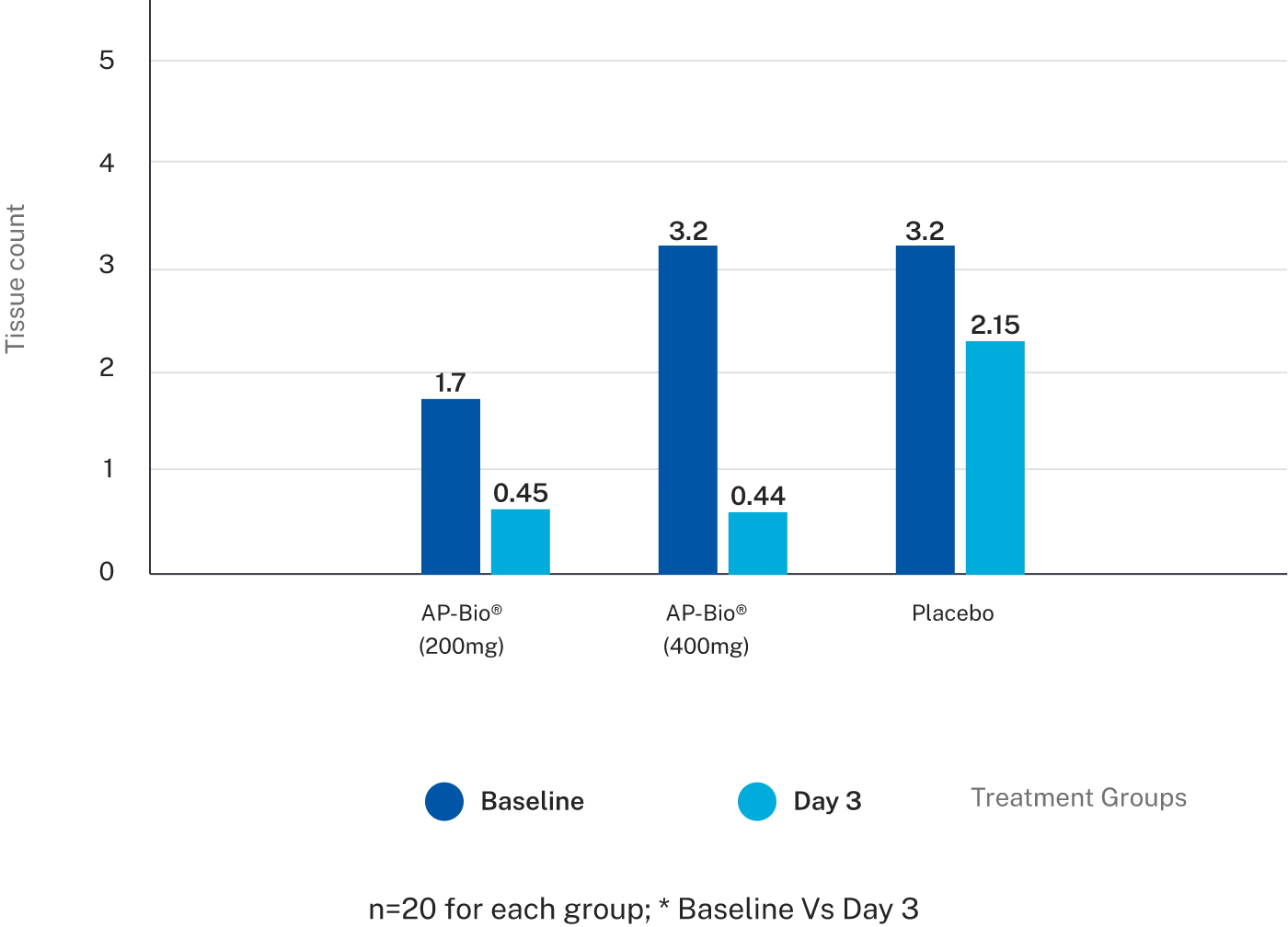
The objective parameters like nasal mucosal weight, number of tissue papers used showed a strong trend towards significant difference in both the AP-Bio® groups when compared to the placebo group.
Reduction in common cold symptoms
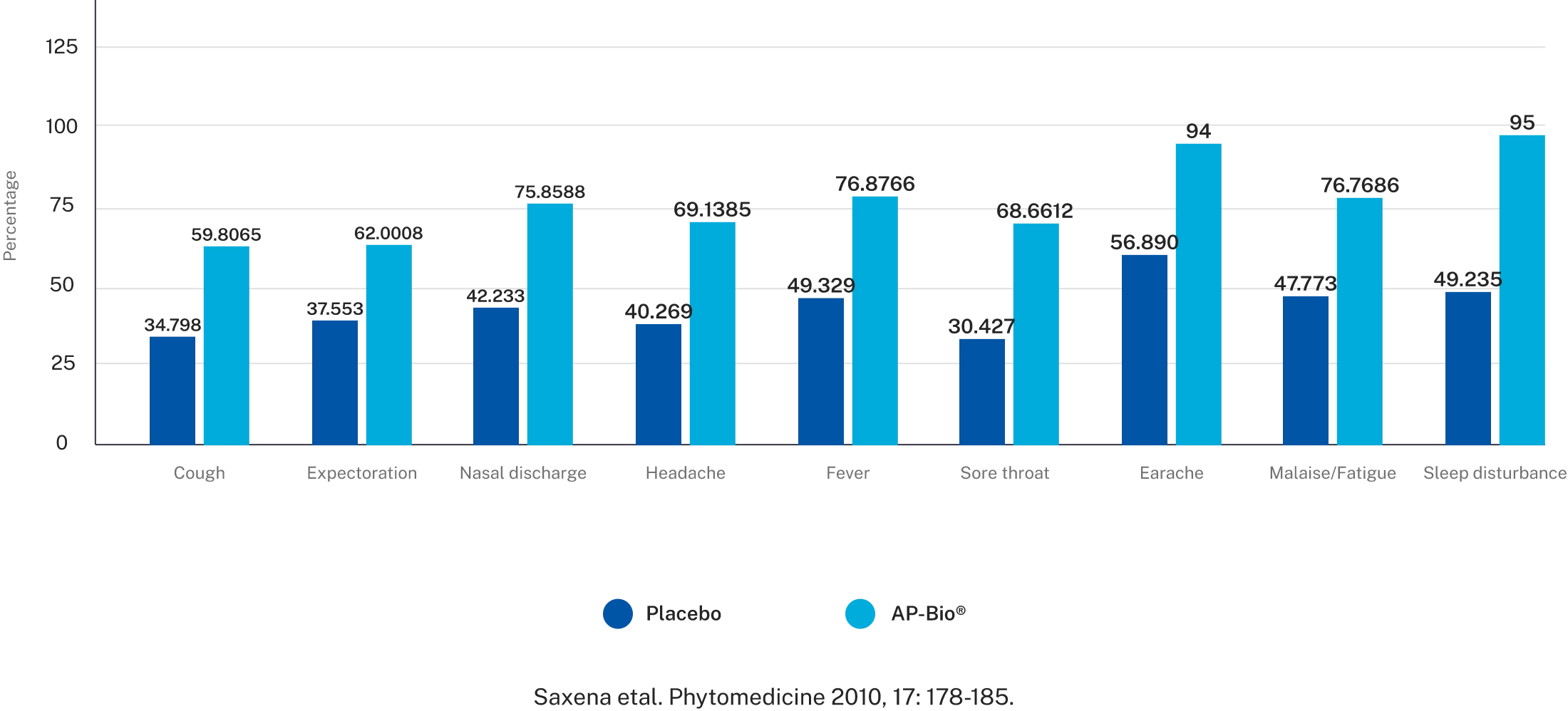
Overall symptoms score
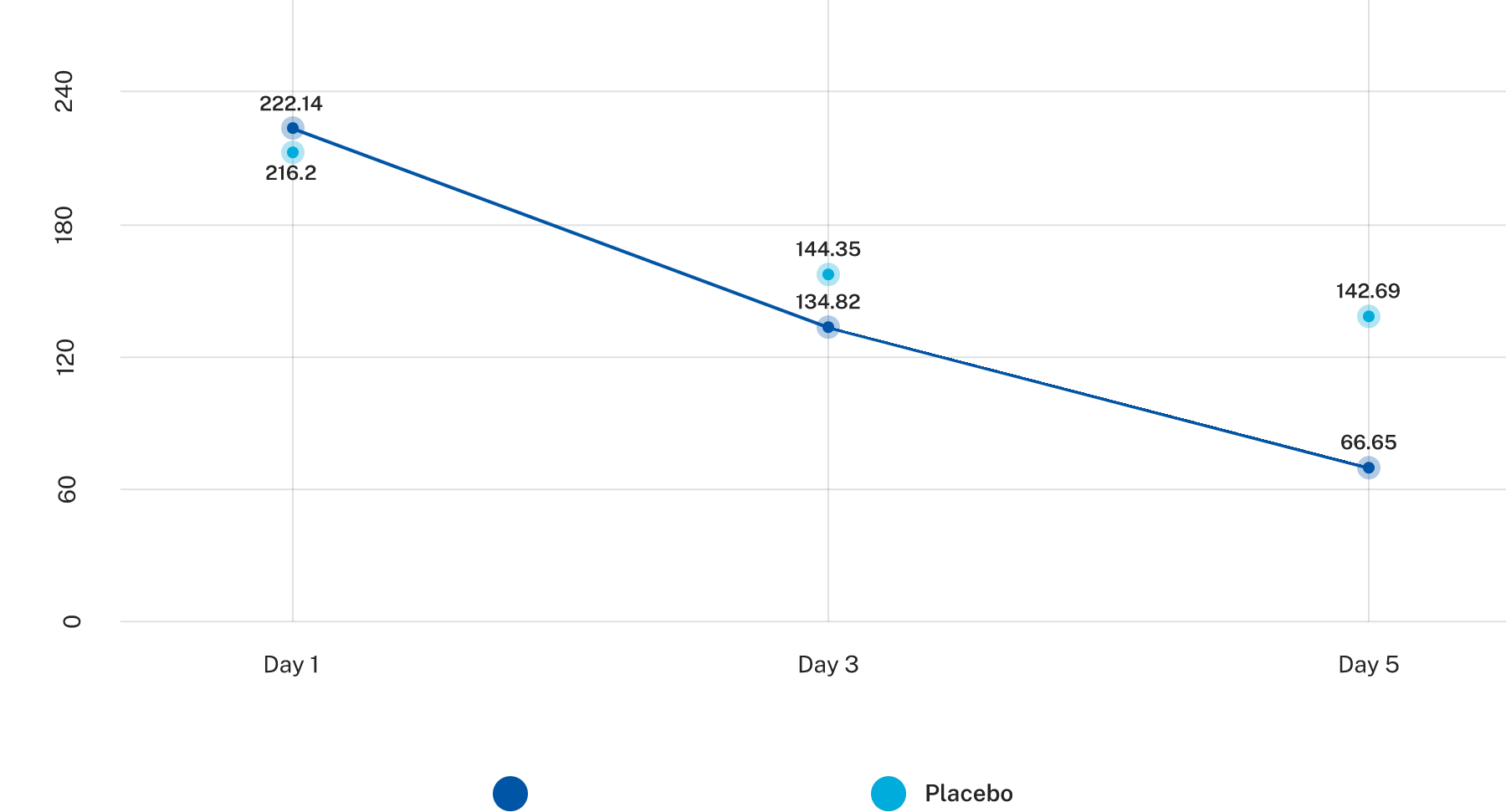
AP-Bio® showed 2 times efficacy as compared to placebo and was well tolerated
- Results- Effect on immune cells
- Results- Effect on immune cell mediators
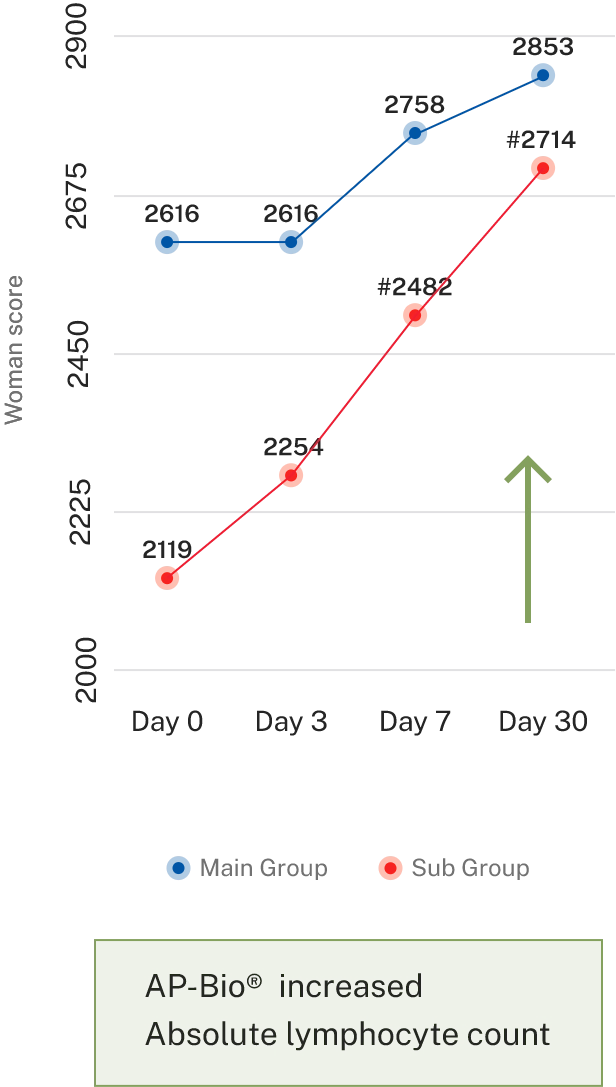
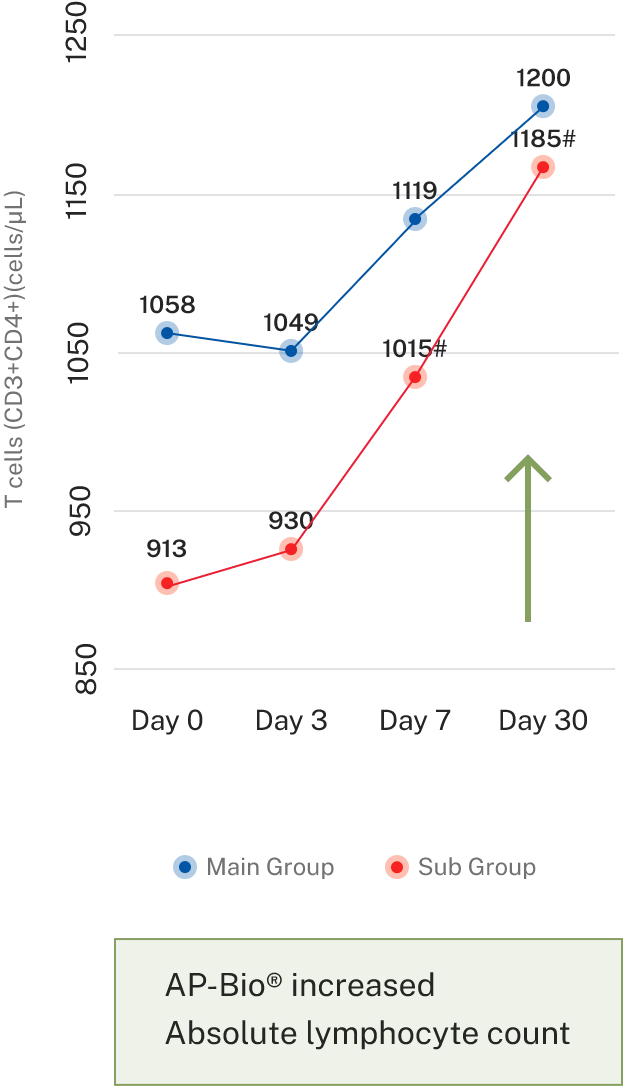
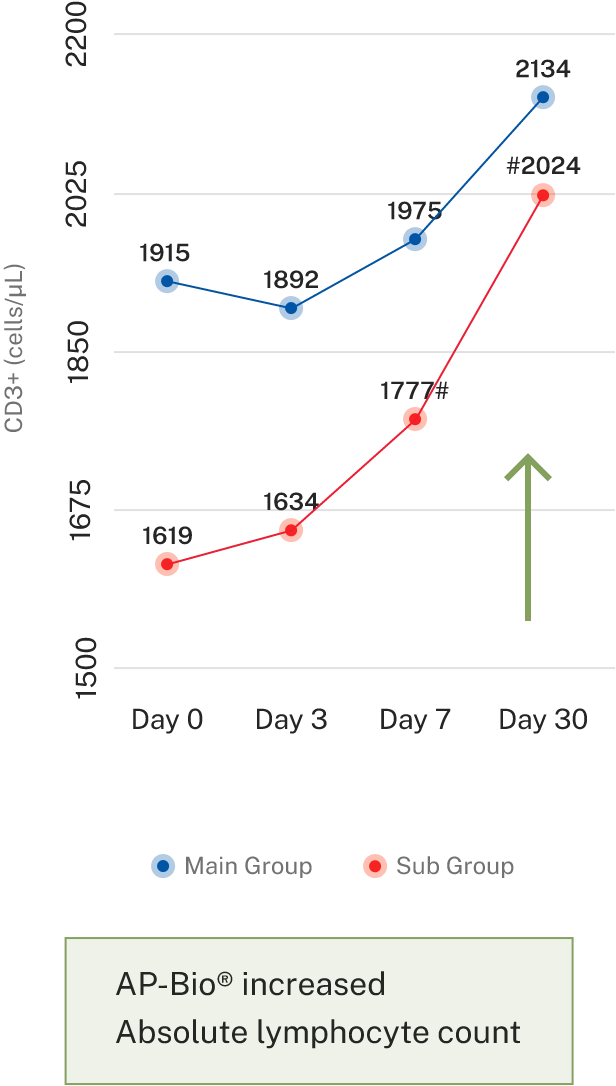
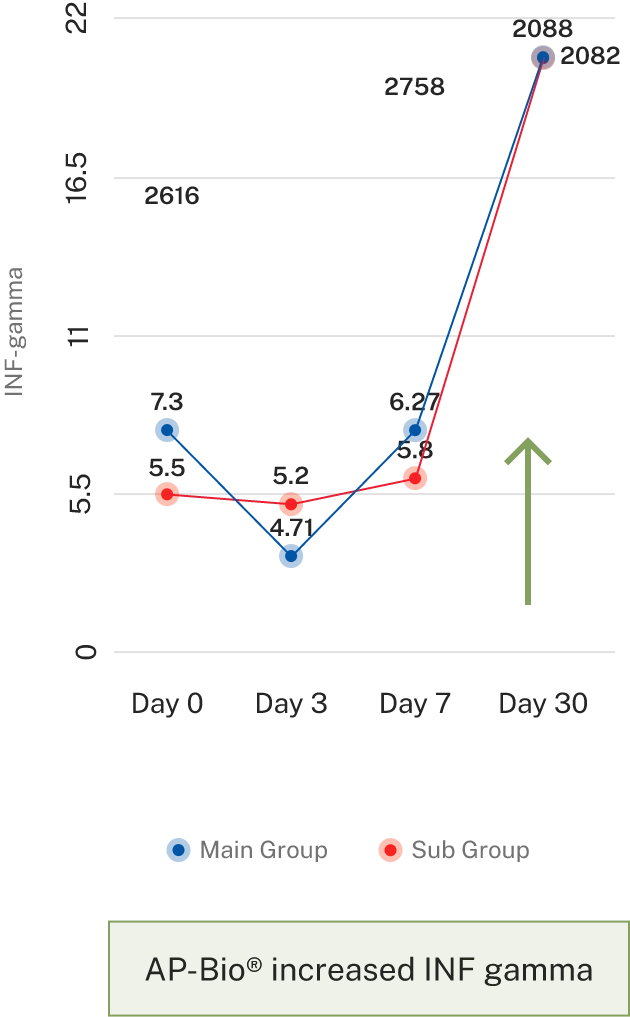

Overall symptoms score
Outcome
Reduced symptoms 55% faster than placebo
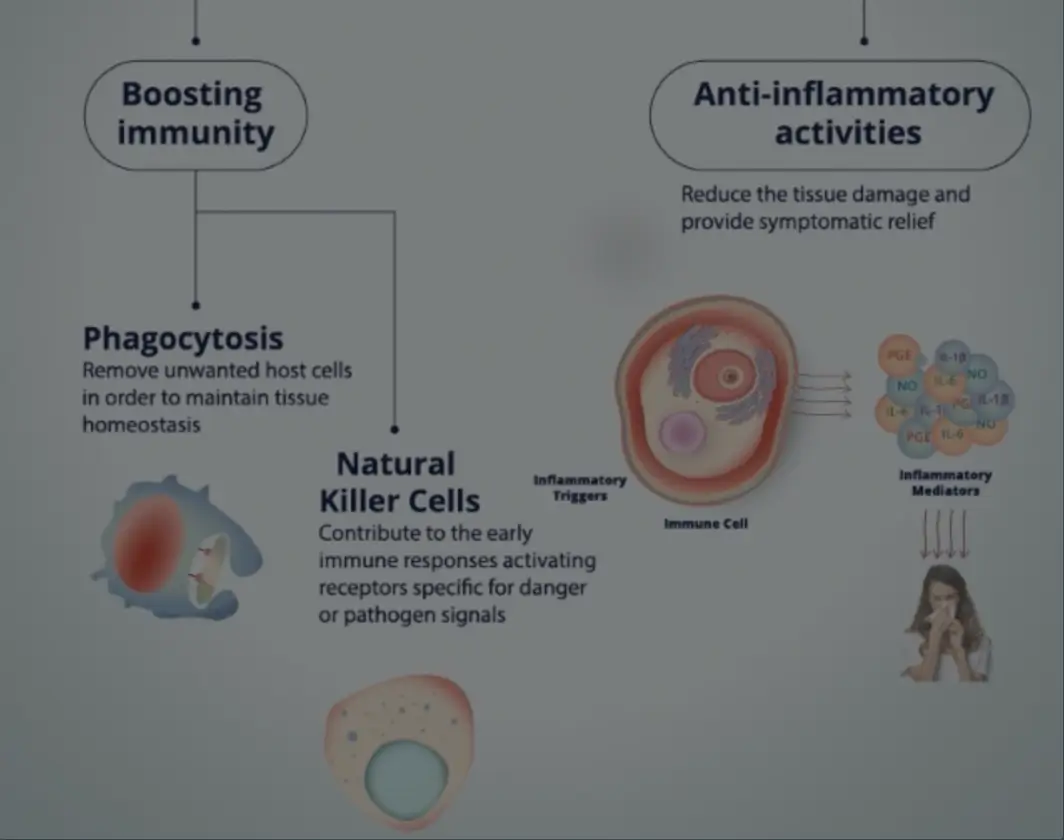
Mechanisms of Action

AP-Bio®: Sustainably making a difference at every step
AP-Bio® is a renewable resource as it is made from sustainable Andrographis paniculata. The process of cultivating Andrographis paniculata for AP-Bio® has always been one that keeps renewability of resources – including human – at the forefront to ensure consistent high-quality yields.
Read MoreWe minimize our carbon footprint by cultivating mostly with non-fossil fuels and without machinery that uses the fossil fuels. We reuse the water used for growing the crop. During manufacturing/processing, the majority of the power utilized is from hydro and solar energy sources. All the wastes from the manufacturing facility are disposed responsibly as per statutory norms. We are an ISO14001-certified (environmental management) organization, and our manufacturing facility is surrounded by trees to provide a green belt.
We are proud of our clean label attributes – AP-Bio® is USP compliant for pesticide residue and heavy metals,non-irradiated, NON-GMO project-verified, and is certified both Kosher and Halal. AP-Bio® is manufactured under strict global guidelines of safety and quality control. Its manufacturer, Natural Remedies, has achieved ISO 22000, ISO 9001 and OHSAS 18001 certification from Bureau Veritas, is NSF GMP-certified, USFDA inspected.

Health Claims*
The human clinical study on AP-Bio® allows for the following health claims:
- Austrailia
Support immune function and to relieve symptoms of mild fever, common cold and sore throat


Dosage:
200mg – 400mg per day

AP-Bio®: Stands for things that matter to you
Formulations possible with AP-Bio®

Tablets

Capsules

Watch the AP-Bio® video to learn more.
AP-Bio® - Fast acting Andrographis paniculata to support Immune health
Why choose AP-Bio®?
7 unique bioactives
Faster onset of action
Low dose allows flexibility in formulation
NON-GMO project verified
Sustainable supply chain
More than 800 Million doses sold worldwide
3 clinical studies on immunity Clinically researched to work by healthy inflammatory response & immuno-stimulatory balance
Relevant & scientifically validated claims on stress & sleep