



Traditional uses
Bacopa monnieri has been relied upon in India by Ayurvedic medical practitioners for nearly 3,000 years. Ayurvedic practitioners have classified Bacopa as a ‘medhyarasayana, a treatment for improving memory and intellect (‘medhya’).
Additionally, the herb has been mentioned in several ancient Ayurvedic treatises including the Charaka Samhita (6th century AD) and, about 1,000 years later, the Bravprakash Var-Prakarana (16th century AD).
As Brahmi, Bacopa has been utilized in traditional Ayurveda as a nerve tonic to improve learning, memory, and concentration, as well as to relieve anxiety and support against
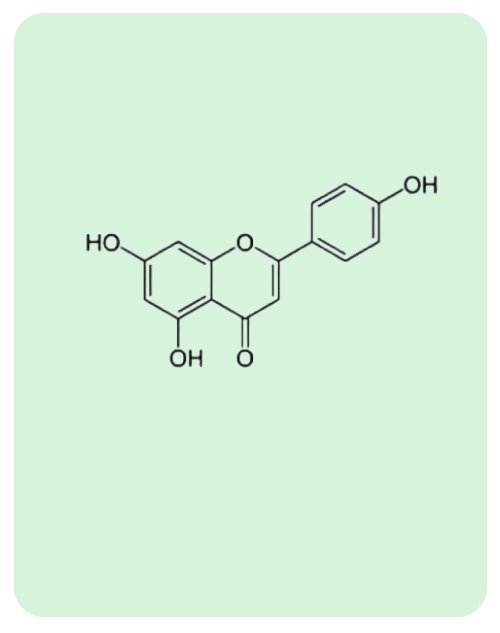
BacoMind®: 9 unique bioactives
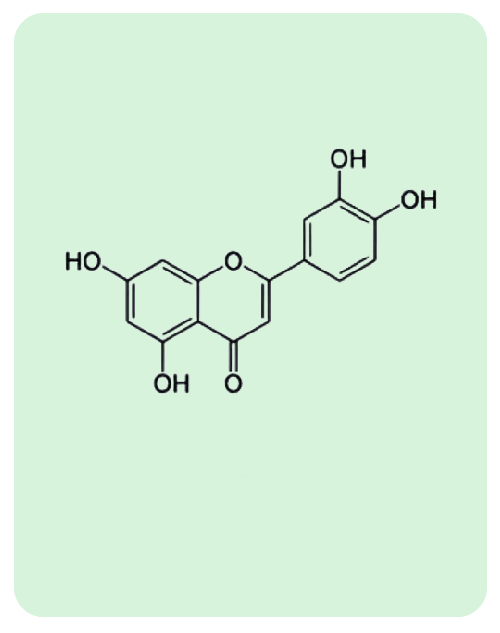
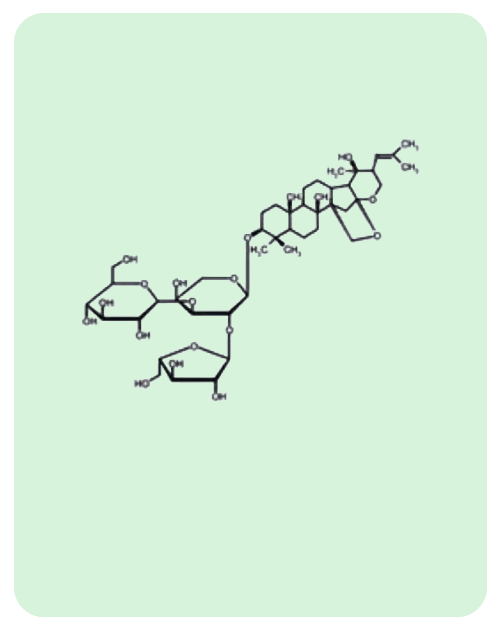
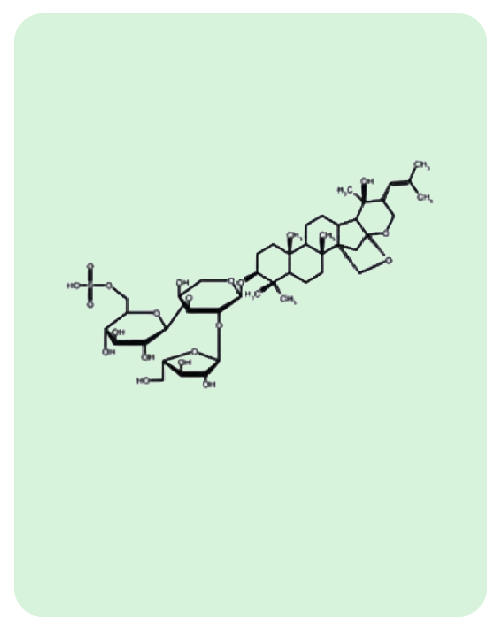
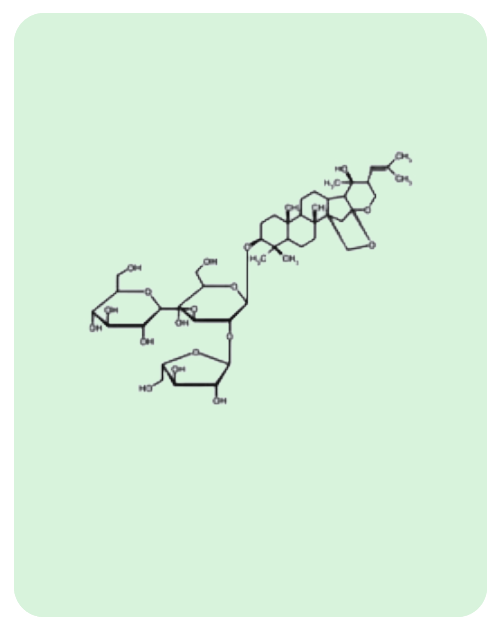
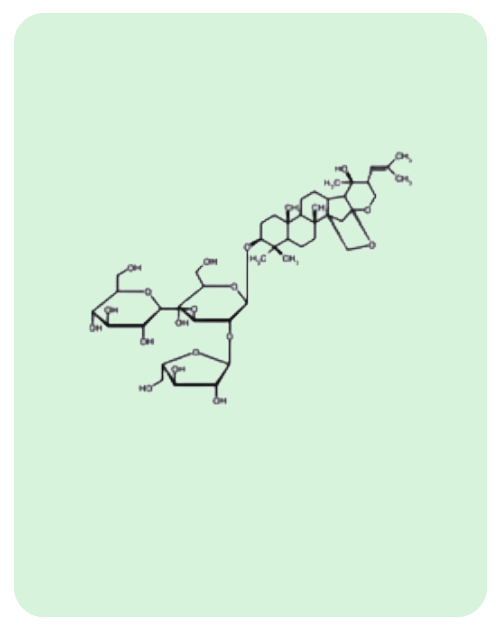
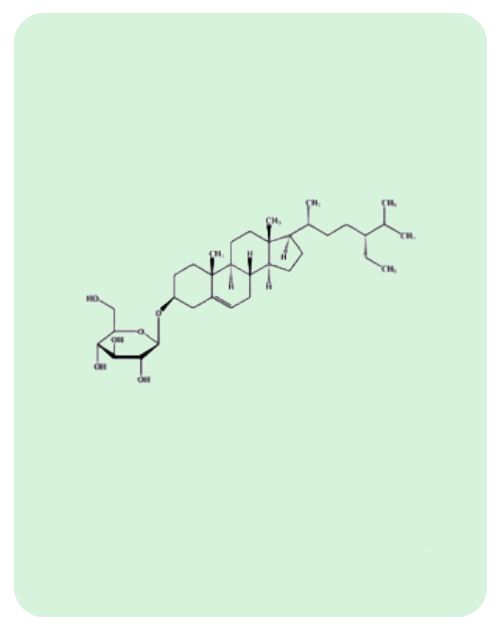
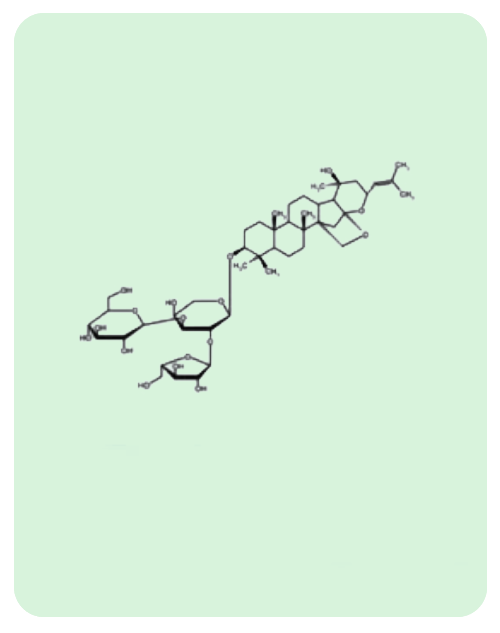
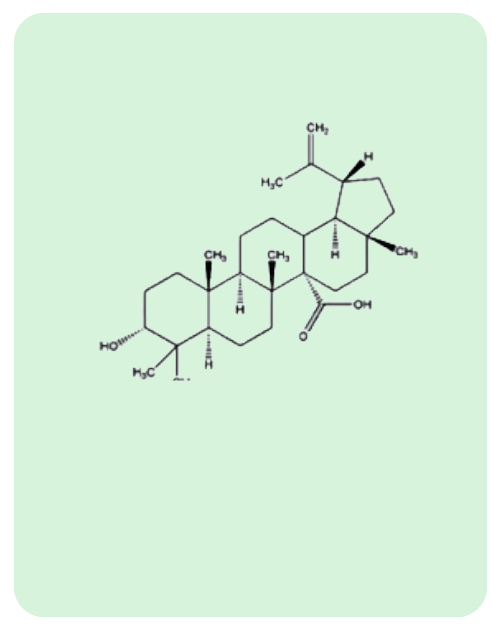











Traditional uses
Bacopa monnieri has been relied upon in India by Ayurvedic medical practitioners for nearly 3,000 years. Ayurvedic practitioners have classified Bacopa as a ‘medhyarasayana, a treatment for improving memory and intellect (‘medhya’).
Additionally, the herb has been mentioned in several ancient Ayurvedic treatises including the Charaka Samhita (6th century AD) and, about 1,000 years later, the Bravprakash Var-Prakarana (16th century AD).
As Brahmi, Bacopa has been utilized in traditional Ayurveda as a nerve tonic to improve learning, memory, and concentration, as well as to relieve anxiety and support against
Clinical study 1
Clinical study 2
Clinical study 1
A randomized double-blind placebo-controlled study in elderly Australians for memory
Clinical study 2
A randomized double-blind placebo-controlled
Study in elderly Australians for memory
Condition
Healthy elderly
individuals
Participants
98 elderly
individuals (Age: ≥55 years)
Dose
300 mg per day
Duration
12 weeks
Evaluation
RAVLT Objective at 12 week (RAVLT : Rey Auditory verbal Learning Test)
Outcome
BacoMind® improved memory acquisition and retention
Clinical study 3
Clinical study 4
Clinical study 5
Clinical study 3
An open labeled clinical study in children requiring IEP
Clinical study 4
Open labeled study in children with ADHD
Clinical study 5
A randomized, open label, dose escalation safety study in healthy adults
An open labeled clinical study in
Children requiring IEP
Condition
Children requiring Individual Education Program (IEP)
Participants
24 children (Age: 4-18 years)
Dose
225 mg per day
Duration
4 months
Evaluation
At 4 months
Open labeled study in Children with ADHD
Condition
ADHD (Attention Deficit Hyperactivity Disorder
Participants
27 children (Age: 6-12 years)
Dose
225 mg per day
Duration
6 months
Evaluation
At 6 months
A randomized, open label,
Dose escalation safety study in healthy adults
Condition
Healthy adults
Participants
23 elderly individuals (Age: 18-45 years)
Dose
300 mg per day for 15 days continued with 450 mg per day for next 15 days
Duration
30 days
Evaluation
Pre and post treatment
Health Claims
The human clinical study on BacoMind® allows for the following health claims:
Helps to improve cognitive function
and memory during aging
retention in healthy older people
Helps to improve acquisition
and retention in healthy older people
Helps to improve functions such…

Helps to improve cognitive functions such
as focus and verbal memory in the elderly
Supports brain health

Supports brain health

Access
The power of 9
Bioactives
Exploring the Hidden
Potential of Bacopa
for Brain Health
Download our whitepaper to get answers to all
your questions about enhancing gnitive health
and wellness

BacoMind® for Cognitive Health
Exploring the Hidden
Potential of Bacopa
for Brain Health
Download our whitepaper to get answers to all
your questions about enhancing gnitive health
and wellness
