

Innovation center
Natural Remedies R&D Center caters to both Animal Health care business as well as Human Health Care business and provides a platform for developing innovative products.
Natural Remedies R&D Centre is well equipped with latest instruments to ensure and maintain the world class standards of health and safety, improved efficacy and increased productivity.

Milestones

Registered patents
Scientists with various domain expertise
Monographs, contributed to US & Indian Pharmacopeia
Scientific publications in peer-viewed journals
Phytocompounds isolated for global reference standards
Our Innovation Departments
- Pharmacology & Toxicology
- Analytical & Instrumentation
- Formulation & Development
- Phytochemistry
- Bioassay
- Microbiology
- Agronomy

Profile
The department comprises three major divisions: In vitro, In vivo, and clinical pharmacology. Presently, the department includes a team of competent scientists from various disciplines like pharmacology, biochemistry, and other allied life sciences. The department is committed to the development of a strong herbal research environment within and between various other divisions. The divisions are well equipped with sophisticated instruments for in vitro pharmacological assays and a central animal facility registered under Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) for efficacy and toxicity studies.
Mandate
Evaluation of herbs for therapeutic use and safety assessment of mono- and poly-herbal formulations are the core areas of focus. The team is engaged in evaluating a range of products for veterinary and human health care, to cater to the needs of livestock and human segments.
Areas of Competence
- Inflammation
- Pain
- Diabetes
- Weight management/obesity

Instrumental analysis is an integral part of quality assurance. The department comprises three major divisions: In vitro, In vivo and Clinical Pharmacology. Presently, the department includes a team of competent scientists from various disciplines like pharmacology, biochemistry, and other allied life sciences. The department is committed to the development of a strong herbal research environment within and between various other divisions. The divisions are well equipped with sophisticated instruments for in vitro pharmacological assays and a central animal facility registered under Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) for efficacy and toxicity studies.
UV-VIS Spectrophotometer
High Performance Liquid Chromatography (HPLC), Preparative & Analytical
Gas Chromatograph
HPTCL
FTIR
We also have access to Mass spectrometer, LC-MS, NMR, AAS, ICP-MS, etc. This group is already engaged in development of analytical monographs for more than one dozen Indian medicinal plants for the forthcoming Indian Pharmacopoeia along with Central Indian Pharmacopoeia Laboratory (C I P L) (Govt. of India), Ghaziabad. This lab routinely uses specific and non-specific bioassays wherever possible in order to achieve bio-standardization.
Mandate
Evaluation of herbs for therapeutic use and safety assessment of mono and poly-herbal formulations are the core areas of focus. The team is engaged in evaluating a range of products for veterinary and human health care, to cater to the needs of livestock and human segments.
Areas of Competence
Development of meaningful quality assessment parameters for plants where actives / bioactives are not known
Quantitative determination of known active principles, biomarkers, and marker components in raw material, extracts, essential oils using HPLC, HPTLC, GC, etc
Development and validation of analytical procedures
Residual analysis (heavy metals, mycotoxins, pesticides, and solvents)
Stability testing
Standardization of herbal products

Formulation and development is one of the key departments in R&D. The main objective of this department is to develop new formulations in different dosage forms for human and veterinary use. The lab is equipped with basic infrastructure for carrying out formulation studies. The lab has developed the first herbal aerosol spray for veterinary use in India.
Areas of Competence
Development of appropriate dosage forms which are stable and convenient to administer
Preformulation studies to understand the nature of actives from formulation angle
Stability studies of finished formulations for establishing the shelf life<
Conducting scale up trials to produce standard operating procedures for commercial production
End to end management of new product innovation in collaboration with other labs
Application of best in practices for new product development (example: Stage Gate Approach)

The Phytochemistry laboratory develops commercially viable processes for the optimum extraction of medicinal plants where active constituents (markers) are known. The plant extracts generated thereof are standardized to contain known amount(s) of biologically active constituent(s). For plants where active / markers are not known, bioactivity guided fractionations are undertaken and active / bio-markers are isolated for the purpose of standardization.
Chromatographic techniques like column, flash, MPLC, Preparative HPLC are employed for isolating constituents. The isolated single chemical entities are thoroughly characterized using the conventional spectroscopic (UV, IR, NMR & Mass spectroscopy), and chromatographic techniques.
Areas of Competence
Identification of crude drugs using pharmacognostic techniques
Isolation of markers for standardization of herbal products
Bioactivity guided fractionation of herbs to isolate their bio-active compounds
Development of extraction procedures for medicinal plants
Isolation of phytochemicals of high purity for use as chromatographic reference standards
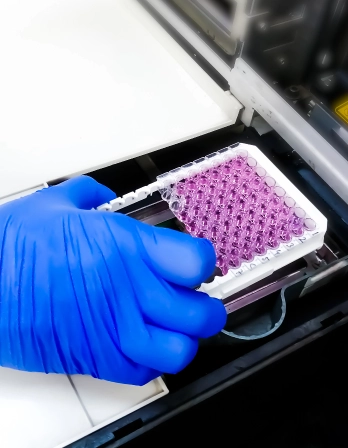
In the discovery cycle, for the predictions of the effects that a drug will display, and measurement of its specific functional response induced in a biological system, a ‘bioassay’ is required. A bioassay is thus an essential tool for research and development. Bioassays based on the in vitro responses of enzymes or stable cell lines are commonly the assay systems of choice for this purpose. These bioassays are rapid, reproducible, economical, and sample hungry.
We have standardized and routinely perform more than 125 in vitro assays relevant to the selection and optimization of new drug candidates using specialized assay technologies based on different detection modes like Absorbance (colorimetric, ELISA), Luminescence, and Fluorescence (Fluorescence Intensity, Fluorescence Polarization, Time-Resolved Fluorescence, etc) in 96 and 384 well formats.
The simple, rapid, and cost-effective nature of bioassays facilitates the screening of a large number of samples. Plant’s effective in in-vitro assays are developed into prototypes, which ultimately get tested in in vivo assays.
Apart from new product development, bioassays can be effectively used for screening campaigns, bioactivity-guided fractionation, compatibility studies, and stability studies.
Areas of competence
Development of in vitro pharmacological methods for drug evaluation
Biological standardization of herbal products
Application of bioassay methods to stability testing and testing combinational effect of herbal extracts

This laboratory is specialized to conduct microbiological quality control and antimicrobial screening of herbal products. Various analytical procedures in this laboratory are tailor-made to suit herbal products and are in accordance with the USP method.
Areas of Competence
Analysis of herbal products for microbiological contamination
Determination of MIC
Determination of MBC
Immersion bioautography for identification of antibacterial compounds.
Preservative efficacy testing
Development of preservative systems
Testing of plant extracts and their derivatives for combinational effects to determine synergy, antagonism and additivity

As a manufacturer of herbal products, we consume more than 500 tonnes of crude drug / annum. This requirement of crude drugs is almost wholly fulfilled by collections from the wild / forests. But it is not possible to derive all this material from the wild, because the population of wild medicinal plants is growing thinner day by day due to over exploitation and indiscriminate deforestation. Moreover, because of insufficient raw material (poor availability), the price of material shoots up. These are thus valid reasons for cultivating medicinal plants.
We undertake contract farming of selected Indian medicinal plants through interested farmers and offer cent percent buy back. Our agriculture farming is stipulated under strictly defined conditions as are specific for each plant. We provide the planting material and complete cultivation plan to farmers and to supervise the proper implementation of the plan we have groomed a team of agriculture graduates. Our agronomy team has covered approximately 1,500 acres of land under contract farming in South India through buy-back agreements.
Through contract farming we have done good R&D work to formulate Good Agricultural Practices, which includes proper selection and identification, propagation methods, cultivation techniques, harvesting, step-wise quality control of raw material up to processing stage, post-harvest treatment, storage, and safety. We provide buy-back guarantee to farmers who can grow medicinal plants of our interest.
For more details contact: [email protected]
Quality & Assurance
In addition to providing the traditional values of reproducibility, accuracy, efficient timelines, and quality reporting, our contractual services are characterized with:

Custom study design and data interpretation by highly experienced scientists

Collaborative approach during the scientific process to meet the study objectives

Capability to develop new models-fulfill unmet needs
Natural Remedies is a recognized leader in the field of herbs and phytocompounds, as proven by accolades received from organizations and personnel from across the world. The analytical laboratory has contributed many monographs on Indian medicinal plants to various national and international pharmacopoeia.
Probiotic bacteria
Biochemical / enzyme inhibition assays are biological methods through which the bioactivity of test substances can be assessed
Standardized / custom programs of preclinical discovery solutions to meet the needs of the
pharmaceutical, natural product, and biotechnology industries.
ADME assay
As researchers try to predict the absorption, distribution, metabolism, and excretion (ADME) characteristics of a compound, the advent of various in vitro high throughput assays has come as a boon. The liver is the central organ in ADME. ㅤ

Contractual Services
Bioactivity Guided Fractionation
Bioassay Servicees
Biochemical assay
Cell assay
ADME assay

Contractual Services
Identification and Fingerprinting
Quantification of Phytoconstituents
Physiochemical Analysis
Multi Residual and Pesticide Analysis
Microbiological Analysis

Training programme modules
One Week Training Module
Two Week Training Module
One Month Training Module

We are a leading group in the field of phytochemistry in India. Our dedicated team of phytochemists are involved in isolating bioactive phytochemicals from different medicinal plants. We have been supplying phytochemical reference substances for over a decade now.
Certifications







